


One of Nature's most basic rules:
Systems naturally seek their lowest available energy state - or - logs
roll downhill
- So, left alone, hydrogen atoms tend to be in the ground state.
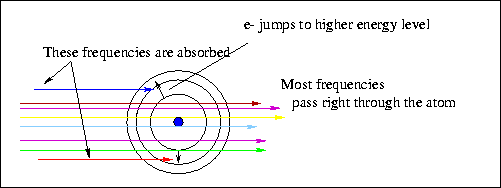
Now, let's bombard an H atom with photons. What happens? Most
of the photons go zipping right past without interacting with the
H atom. But! photons with just the RIGHT energy get absorbed by the
atom
Right means that the energy of the photon corresponds to the
energy level difference between ``allowed'' orbits in the H atom.
Absorbed means that the energy of the photon will now be gone
and the atom will be in a higher energy state.
- A photon with frequency
 will be absorbed
by an atom if the energy of the photon corresponds to an energy level difference
between allowed states in the atom
will be absorbed
by an atom if the energy of the photon corresponds to an energy level difference
between allowed states in the atom
- What happens next? Remember, Nature seeks the lowest available energy level so
the
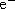 bumped to an excited orbit will drop back to the ground state.
bumped to an excited orbit will drop back to the ground state.
- Another Law of Nature, Conservation of Energy , states that the energy
difference between the excited state and ground state must appear somewhere when the
atom makes the transition. ``Somewhere'' is as a photon with the energy of the
original one.
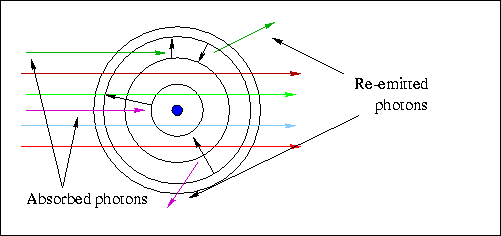
- Below is a schematic diagram of the allowed orbits in a Hydrogen
atom. If you can answer the questions about it, you've got the idea.
- Which transition(s) correspond(s) to the absorption of a photon?
- Which transition corresponds to the highest energy photon emitted ?
- Which transition corresponds to the shortest wavelength photon emitted?
- Which transition corresponds to the higest frequency photon emitted?


 Michael Bolte
Michael Bolte
Thu Jan 15 10:40:14 PST 1998


