- Suppose the Earth's orbit was not tilted with respect to its
orbital plane. True or False?
We would no longer experience Day and Night --- False
We would see the same stars in the night sky all year around --- False
We would no longer experience changes in the seasons -- True
- Given that the Earth is around 24,000 miles in circumference
and the speed of light is 186,000 miles/sec; how long would it take a photon
to circle the Earth?
Suppose that you have  .
.
Try #1:
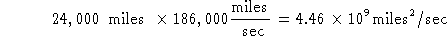
Units of miles  /sec make no sense.
/sec make no sense.
Try #2:
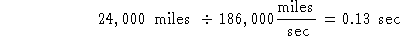
This has the right units and is the right answer.
- Given Wien's Law:
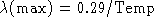
(a) How would you manipulate this equation to allow you to solve for the
temperature of a solid if you are told the radiation from the object peaks
at 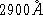 ? (
? ( 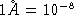 cm)
cm)
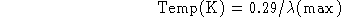
(b) What is the temperature?
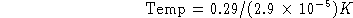
- For each of the observers below, label whether they measure a Redshift, Blueshift
or Noshift.
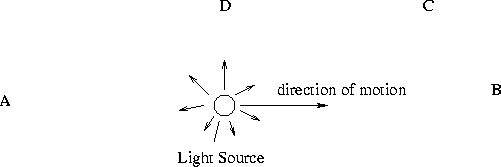
- A -- reshift
- B -- blueshift
- C -- blueshift (smaller than B)
- D -- noshift
- What time does the new moon rise? -- sunrise
- Rank the following in order of increasing wavelength (1 - shortest; 6 - longest):
1 X-rays
2 Blue light
3 Red light
4 Infrared
5 FM radio (800 MegaHertz)
6 AM radio (800 kiloHertz)
- What type of spectrum would you expect to see from a gas
with all the atoms in their ground state and no background source of radiation?
No spectrum at all -- for the atom to produce light (at least as far as
we have discussed it in this class) you need to have some electrons in excited
states so a photon can be emitted on de-excitation
- Suppose the Earth's atmosphere was twice as ``thick'' (that is twice
as many molecules and atoms between sea level and the top of the atmosphere)
as it is now. The
color of the Sun at noon (compared to the real case of our current atmosphere) would
be:
a) unchanged b) bluer c) redder
- Suppose that electron orbits in hydrogen atoms were not
``quantized'', but the electron in a hydrogen atom could occupy
any orbit with arbitrary energy. What type of spectrum
would you expect from a hot gas of this hypothetical hydrogen?
A continuous spectrum
![]() .
.
![]()
![]() /sec make no sense.
/sec make no sense.
![]()
![]() ? (
? ( ![]() cm)
cm)
![]()
![]()
